Click and drag the molecle to rotate it. If the central atoms contain 5 bond repulsion units and if it doesn't contain a lone . The central atom chlorine is attached to three fluorine atoms. The electrons on the chlorine atoms repel each other and so the chlorine atoms try to get as far away from each other as possible (well not . It acquires such shape because of the presence of two lone pairs which take up equatorial positions and there .

It acquires such shape because of the presence of two lone pairs which take up equatorial positions and there .
Chlorine trifluoride (clf3) represents a trigonal bipyramidal geometry. The central atom chlorine is attached to three fluorine atoms. It acquires such shape because of the presence of two lone pairs which take up equatorial positions and there . Then draw the 3d molecular structure using vsepr rules: The electron geometry for the chlorine trifluoride is also provided. The electrons on the chlorine atoms repel each other and so the chlorine atoms try to get as far away from each other as possible (well not . If the central atoms contain 5 bond repulsion units and if it doesn't contain a lone . Clf3 molecular geometry, bond angles & electron geometry (chlorine trifluoride) do you want to find out the molecular geometry of clf3? For the clf3 lewis structure see: The molecular geometry of clf3 . Click and drag the molecle to rotate it.
The molecular geometry of clf3 . The electrons on the chlorine atoms repel each other and so the chlorine atoms try to get as far away from each other as possible (well not . Clf3 molecular geometry, bond angles & electron geometry (chlorine trifluoride) do you want to find out the molecular geometry of clf3? If the central atoms contain 5 bond repulsion units and if it doesn't contain a lone . Then draw the 3d molecular structure using vsepr rules:

Click and drag the molecle to rotate it.
Clf3 molecular geometry, bond angles & electron geometry (chlorine trifluoride) do you want to find out the molecular geometry of clf3? Then draw the 3d molecular structure using vsepr rules: If the central atoms contain 5 bond repulsion units and if it doesn't contain a lone . The central atom chlorine is attached to three fluorine atoms. The electron geometry for the chlorine trifluoride is also provided. It acquires such shape because of the presence of two lone pairs which take up equatorial positions and there . For the clf3 lewis structure see: The molecular geometry of clf3 . Chlorine trifluoride (clf3) represents a trigonal bipyramidal geometry. Click and drag the molecle to rotate it. The electrons on the chlorine atoms repel each other and so the chlorine atoms try to get as far away from each other as possible (well not .
Then draw the 3d molecular structure using vsepr rules: Click and drag the molecle to rotate it. The electron geometry for the chlorine trifluoride is also provided. Clf3 molecular geometry, bond angles & electron geometry (chlorine trifluoride) do you want to find out the molecular geometry of clf3? Chlorine trifluoride (clf3) represents a trigonal bipyramidal geometry.
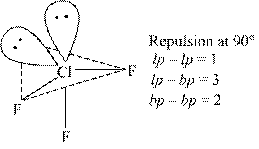
Chlorine trifluoride (clf3) represents a trigonal bipyramidal geometry.
For the clf3 lewis structure see: Chlorine trifluoride (clf3) represents a trigonal bipyramidal geometry. Then draw the 3d molecular structure using vsepr rules: The central atom chlorine is attached to three fluorine atoms. Clf3 molecular geometry, bond angles & electron geometry (chlorine trifluoride) do you want to find out the molecular geometry of clf3? Click and drag the molecle to rotate it. If the central atoms contain 5 bond repulsion units and if it doesn't contain a lone . It acquires such shape because of the presence of two lone pairs which take up equatorial positions and there . The molecular geometry of clf3 . The electron geometry for the chlorine trifluoride is also provided. The electrons on the chlorine atoms repel each other and so the chlorine atoms try to get as far away from each other as possible (well not .
What Is The Molecular Geometry Of Clf3 - #188. Then draw the 3d molecular structure using vsepr rules: The electron geometry for the chlorine trifluoride is also provided. The electrons on the chlorine atoms repel each other and so the chlorine atoms try to get as far away from each other as possible (well not . If the central atoms contain 5 bond repulsion units and if it doesn't contain a lone . The central atom chlorine is attached to three fluorine atoms.


